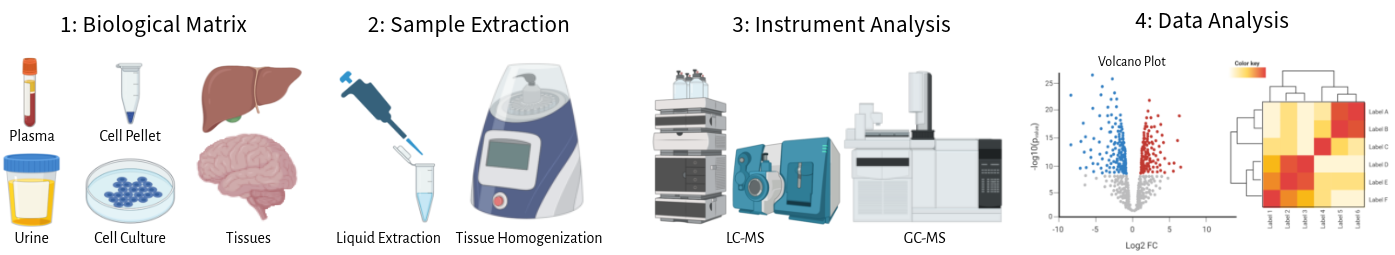
Metabolomics Core Facility
The University of Utah Metabolomics Core uses mass spectrometry as a platform to provide high-quality metabolomics data for the University of Utah researchers, academic collaborators, and commercial applications. The core specializes in LC-MS and GC-MS metabolomics and lipidomics analysis, as well as metabolite quantitation. The core was initiated by a NIDDK funded Center of Excellence in Molecular Hematology and continues to be funded by an NIDDK Cooperative Centers of Excellence in Hematology award. The laboratory is fit with a number of state-of-the-art instruments and staffed by three Ph.D. level scientists and two BS level specialists, with over 40 combined years of mass spectrometry experience.
Due to the chemical diversity of the metabolome, no single platform or extraction method exists. Therefore, the core offers multiple complimentary services: GC-MS targeted and non-targeted metabolomics, LC-MS targeted and non-targeted metabolomics, and metabolite quantification. Non-targeted metabolomics is a preferred way for the discovery of metabolic biomarkers and generating initial hypothesis. Targeted metabolomics is employed to target specific metabolic pathways for hypothesis driven research. Additionally, targeted metabolomics is also employed when more sensitivity is required, or absolute quantitation is needed. Please contact the core before beginning a project for help determining which type of analysis is correct for your research.
The lipidomics division within the University of Utah’s Metabolomics Core is well-equipped with state-of-the-art instrumentation and a team of experts. We provide lipidome profiling services for a wide range of biological samples, including serum, urine, tissues, Drosophila, C. elegans, yeast, and bacteria. Our approach encompasses both targeted and non-targeted analysis methods, utilizing an Agilent 6545A UPLC-QToF-MS for discovery lipidomics, Agilent 6490 QQQ UPLC-MS, and SCIEX 6500 QTRAP QQQ UPLC-MS for precise metabolite quantification. The core facility also offers tailored assays for acyl carnitines and sphingolipids, as well as the capability to create custom, targeted platforms to cater to the unique needs of our researchers and collaborators.
To develop an effective metabolite and lipid profiling strategy please consult with Core staff prior to sample submission. This will determine project feasibility and in some cases a pilot project will be needed. This consultation is necessary to determine which extraction and instrumentation would be optimal for your project. A preliminary quote can/will be generated based upon this consultation.
Project Rates
| Service | CIHD Internal | CIHD External | University of Utah | External Academic | Commercial |
| GC-MS Metabolomics | $40.43 | $62.26 | $69.27 | $106.68 | $138.54 |
| LC-MS Metabolomics | $46.44 | $71.52 | $69.86 | $107.59 | $139.73 |
| Lipidomics | $46.44 | $71.52 | $82.33 | $126.78 | $164.66 |
| Senior Associate Data Analysis cost/hour | $106.09 | $163.17 | $106.09 | $163.17 | $212.18 |
| Associate Data Analysis cost/hour | $54.64 | $84.15 | $54.64 | $84.15 | $109.28 |
| GC-MS Instrument cost/hour | $46.44 | $71.52 | $46.44 | $71.52 | $92.89 |
| LC-MS instrument cost/hour | $52.45 | $80.77 | $52.45 | $80.77 | $104.90 |
Requesting Services
Existing users may login directly to the Resource Scheduling System to schedule or order services. This system is cores-wide and uses University of Utah uNID authentication.
Contact Us
Hours of Operation
9:00 am to 5:00 pm
Monday - Friday
Shipping Address
University of Utah
Metabolomics Core
15 N Medical Drive East, RM A306
Salt Lake City, UT 84112-5650 USA
Major Acknowledgements
- Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, Buhrke K, Ekiz HA, Ost KS, Boudina S, O'Connell RM, Cox JE, Villanueva CJ, Stephens WZ, Round JL. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365(6451). Epub 2019/07/28. doi: 10.1126/science.aat9351. PubMed PMID: 31346040; PMCID: PMC7294966.
- Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, Wilkerson JL, Sweeney CR, Pereira RF, Sumida DH, Maschek JA, Cox JE, Kaddai V, Lancaster GI, Siddique MM, Poss A, Pearson M, Satapati S, Zhou H, McLaren DG, Previs SF, Chen Y, Qian Y, Petrov A, Wu M, Shen X, Yao J, Nunes CN, Howard AD, Wang L, Erion MD, Rutter J, Holland WL, Kelley DE, Summers SA. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386-92. Epub 2019/07/06. doi: 10.1126/science.aav3722. PubMed PMID: 31273070; PMCID: PMC6787918.
- Burch JS, Marcero JR, Maschek JA, Cox JE, Jackson LK, Medlock AE, Phillips JD, Dailey HA, Jr. Glutamine via alpha-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood. 2018;132(10):987-98. Epub 2018/07/12. doi: 10.1182/blood-2018-01-829036. PubMed PMID: 29991557; PMCID: PMC6128084 interests.
- Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, Lee S, Jiang L, Huck I, Kershaw EE, Donato AJ, Apte U, Longo N, Rutter J, Schreiber R, Zechner R, Cox J, Villanueva CJ. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab. 2017;26(3):509-22 e6. Epub 2017/09/07. doi: 10.1016/j.cmet.2017.08.006. PubMed PMID: 28877455; PMCID: PMC5658052.
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS, Rutter J. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96-100. Epub 2012/05/26. doi: 10.1126/science.1218099. PubMed PMID: 22628558; PMCID: PMC3690818.
Recent Acknowledgements
- Zhao J, Ballard C, Cohen AJ, Ringham B, Zhao B, Wang H, Zuspan K, Rebentisch A, Locklear BA, Dahl M, Maschek JA, Cox JE, Joss-Moore LA. Postnatal growth restriction impairs rat lung structure and function. Anat Rec (Hoboken). 2023. Epub 2023/07/29. doi: 10.1002/ar.25297. PubMed PMID: 37515384.
- Zhang S, Williams KJ, Verlande-Ferrero A, Chan AP, Su GB, Kershaw EE, Cox JE, Maschek JA, Shapira SN, Christofk HR, de Aguiar Vallim TQ, Masri S, Villanueva CJ. Acute activation of adipocyte lipolysis reveals dynamic lipid remodeling of the hepatic lipidome. J Lipid Res. 2023:100434. Epub 2023/08/29. doi: 10.1016/j.jlr.2023.100434. PubMed PMID: 37640283.
- Sharma R, Antypiuk A, Vance SZ, Manwani D, Pearce Q, Cox JE, An X, Yazdanbakhsh K, Vinchi F. Macrophage metabolic rewiring improves heme-suppressed efferocytosis and tissue damage in sickle cell disease. Blood. 2023;141(25):3091-108. Epub 2023/03/24. doi: 10.1182/blood.2022018026. PubMed PMID: 36952641; PMCID: PMC10315632 interests. F.V. receives research funding from Silence Therapeutics, Vifor Pharma, and PharmaNutra (none of these are relevant to the current project).
- Miljkovic M, Seguin A, Jia X, Cox JE, Catrow JL, Bergonia H, Phillips JD, Stephens WZ, Ward DM. Loss of the mitochondrial protein Abcb10 results in altered arginine metabolism in MEL and K562 cells and nutrient stress signaling through ATF4. J Biol Chem. 2023;299(7):104877. Epub 2023/06/04. doi: 10.1016/j.jbc.2023.104877. PubMed PMID: 37269954; PMCID: PMC10316008.
- Li Y, Chaurasia B, Rahman MM, Kaddai V, Maschek JA, Berg JA, Wilkerson JL, Mahmassani ZS, Cox J, Wei P, Meikle PJ, Atkinson D, Wang L, Poss AM, Playdon MC, Tippetts TS, Mousa EM, Nittayaboon K, Anandh Babu PV, Drummond MJ, Clevers H, Shayman JA, Hirabayashi Y, Holland WL, Rutter J, Edgar BA, Summers SA. Ceramides Increase Fatty Acid Utilization in Intestinal Progenitors to Enhance Stemness and Increase Tumor Risk. Gastroenterology. 2023;165(5):1136-50. Epub 2023/08/05. doi: 10.1053/j.gastro.2023.07.017. PubMed PMID: 37541526; PMCID: PMC10592225.
- Li F, Lin Z, Krug PJ, Catrow JL, Cox JE, Schmidt EW. Animal FAS-like polyketide synthases produce diverse polypropionates. Proc Natl Acad Sci U S A. 2023;120(38):e2305575120. Epub 2023/09/11. doi: 10.1073/pnas.2305575120. PubMed PMID: 37695909; PMCID: PMC10515154.
- Johnson JM, Peterlin AD, Balderas E, Sustarsic EG, Maschek JA, Lang MJ, Jara-Ramos A, Panic V, Morgan JT, Villanueva CJ, Sanchez A, Rutter J, Lodhi IJ, Cox JE, Fisher-Wellman KH, Chaudhuri D, Gerhart-Hines Z, Funai K. Mitochondrial phosphatidylethanolamine modulates UCP1 to promote brown adipose thermogenesis. Sci Adv. 2023;9(8):eade7864. Epub 2023/02/25. doi: 10.1126/sciadv.ade7864. PubMed PMID: 36827367; PMCID: PMC9956115.
- Hicks KG, Cluntun AA, Schubert HL, Hackett SR, Berg JA, Leonard PG, Ajalla Aleixo MA, Zhou Y, Bott AJ, Salvatore SR, Chang F, Blevins A, Barta P, Tilley S, Leifer A, Guzman A, Arok A, Fogarty S, Winter JM, Ahn HC, Allen KN, Block S, Cardoso IA, Ding J, Dreveny I, Gasper WC, Ho Q, Matsuura A, Palladino MJ, Prajapati S, Sun P, Tittmann K, Tolan DR, Unterlass J, VanDemark AP, Vander Heiden MG, Webb BA, Yun CH, Zhao P, Wang B, Schopfer FJ, Hill CP, Nonato MC, Muller FL, Cox JE, Rutter J. Protein-metabolite interactomics of carbohydrate metabolism reveal regulation of lactate dehydrogenase. Science. 2023;379(6636):996-1003. Epub 2023/03/10. doi: 10.1126/science.abm3452. PubMed PMID: 36893255; PMCID: PMC10262665.
- Ferrara PJ, Lang MJ, Johnson JM, Watanabe S, McLaughlin KL, Maschek JA, Verkerke ARP, Siripoksup P, Chaix A, Cox JE, Fisher-Wellman KH, Funai K. Weight loss increases skeletal muscle mitochondrial energy efficiency in obese mice. Life Metab. 2023;2(2). Epub 2023/05/19. doi: 10.1093/lifemeta/load014. PubMed PMID: 37206438; PMCID: PMC10195096.
Citing Our Facility
Acknowledgments
We would like to thank you for acknowledging the our facility. This recognition allows us to highlight the impact of your work and demonstrates the important contributions of our facility makes to research across the University of Utah. The recognition our core receives from your acknowledgments also aids in receiving grants and further funding for equipment and services we can provide to our users.
Self-Run Services / Instrumentation Usage:
In published papers that used instruments at our facility and notably involved staff members please use the following format:
We acknowledge (facility name) at the University of Utah for use of equipment (insert instrument/service details here), and thank (insert any notable staff member – if desired) for their assistance.
Assisted Services:
In published papers where a staff member assisted you in addition to the requested services please use the following format:
We acknowledge (facility name) at the University of Utah for use of equipment (insert instrument/service details here), and thank (insert staff member-required) for their assistance in (service provided).
Collaboration:
For publications resulting from collaborations that assisted with the methodologies, planning process and execution of your experiment in addition to equipment usage we require Co-author attribution on your publication for our facility and any staff members who provided substantial contributions to the originating project.